
“We made a big mistake,” said Byram Bridle, a viral immunologist and associate professor at the University of Guelph’s Ontario Veterinary College, during an interview with Canadian radio personality Alex Pierson on May 27.

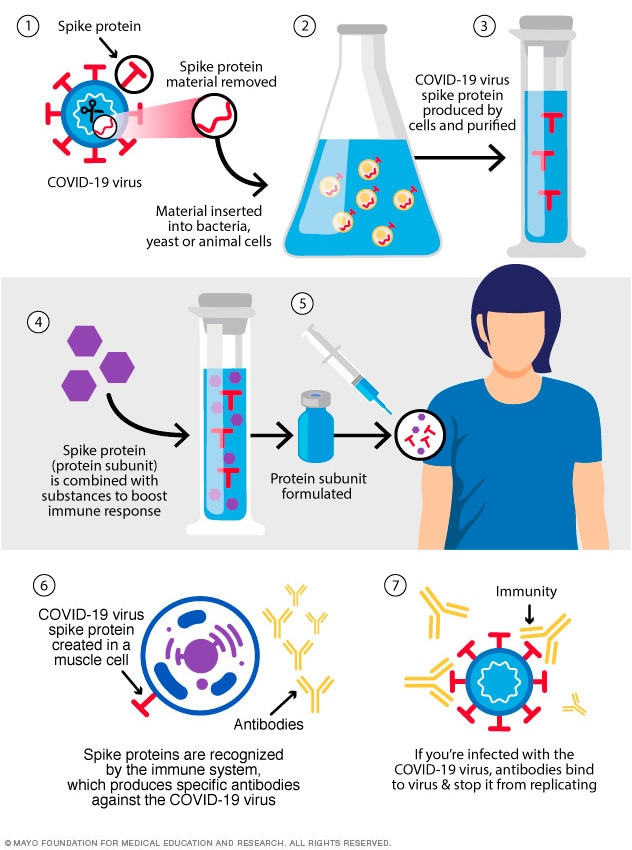
Yet, a Canadian virus immunologist recently claimed that the vaccine’s spike protein is “a pathogenic protein,” “a toxin” that gets into the bloodstream, then accumulates in breast milk and “in a number of tissues” and could lead to cardiovascular and neurological damage in adults, children and infants. Severe adverse reactions to the vaccines have been rare. history” has found that most of the reported side effects after COVID-19 vaccination are minor, such as pain at the injection site or fever. Īccording to the Centers for Disease Control and Prevention, the “most intensive safety monitoring in U.S. These vaccines have been found to be effective and safe in clinical trials with tens of thousands of participants, and in real world conditions with over 325 million doses administered. The process prepares the body against future infection. When the immune system recognizes the proteins, which aren’t normally there, it starts generating antibodies and building an immune response against them. The CDC and FDA vaccine safety monitoring systems, which were expanded for the COVID-19 vaccines and also include a new smartphone-based reporting tool called v-safe, have subsequently identified only a few, very rare adverse events.įor more, see “ How safe are the vaccines? ”Īll of the COVID-19 vaccines currently approved in the United States are designed to instruct human cells to make harmless spike proteins - mimicking a viral protein that’s used by the SARS-CoV-2 virus to enter cells. In the case of the COVID-19 vaccines, randomized controlled trials involving tens of thousands of people, which were reviewed by multiple groups of experts, revealed no serious safety issues and showed that the benefits outweigh the risks. to identify adverse events related to vaccination in near real time. The information is still valuable because it’s a way of being quickly alerted to a potential safety issue with a vaccine, which can then be followed-up by government scientists.Īnother monitoring system is the CDC’s Vaccine Safety Datalink, which uses electronic health data from nine health care organizations in the U.S. There is no screening or vetting of the report and no attempt to determine if the vaccine was responsible for the problem. As its website explains, VAERS “is not designed to detect if a vaccine caused an adverse event, but it can identify unusual or unexpected patterns of reporting that might indicate possible safety problems requiring a closer look.”Īnyone can submit a report to VAERS for any health problem that occurs after an immunization. One key vaccine safety surveillance program is the Vaccine Adverse Event Reporting System, or VAERS, which is an early warning system run by the Centers for Disease Control and Prevention and FDA.
#Protein in vaccine free
In addition, the Food and Drug Administration inspects vaccine production facilities and reviews manufacturing protocols to make sure vaccine doses are of high-quality and free of contaminants. No vaccine or medical product is 100% safe, but the safety of vaccines is ensured via rigorous testing in clinical trials prior to authorization or approval, followed by continued safety monitoring once the vaccine is rolled out to the public to detect potential rare side effects.


 0 kommentar(er)
0 kommentar(er)
